Medical Devices
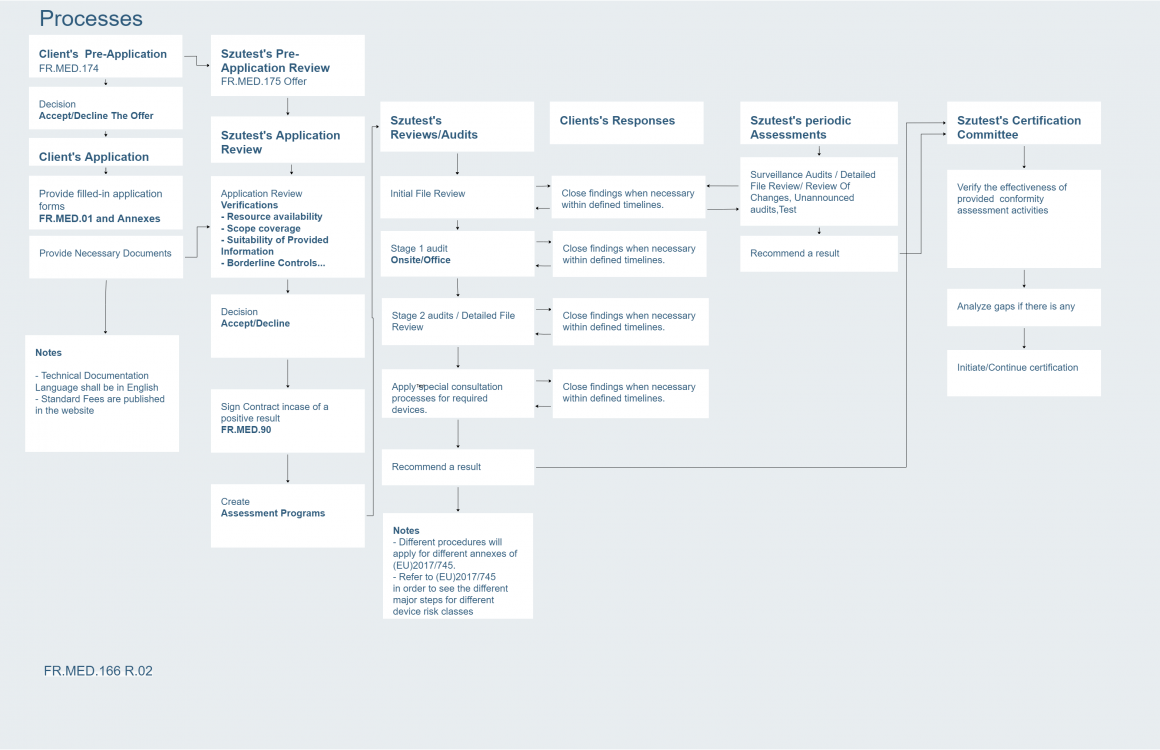
SZUTEST Konformitätsbewertungsstelle GmbH has applied to become a notified body within the framework of the Medical Device Regulation (EU) 2017/745.
WHAT SERVICES DOES SZUTEST OFFER FOR MEDICAL DEVICES?
The scope of the application includes MDR Medical Device Regulation (EU) 2017/745 in Annex IX Part I, Annex IX Part II and Annex XI Part A.
[/cmsmasters_text][/cmsmasters_column][/cmsmasters_row]